Miya Ironami  🌸
🌸
iro_miya@mk.absturztau.be
“Ah yes it’s either a drug user, a tranny, a barista, or a just a huge fucking nerd”
@iro_miya you mean like the vibe or how to decipher the molecule?
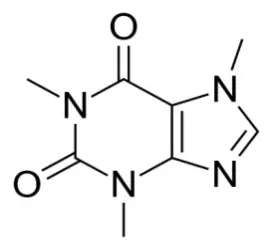
@iro_miya im still studying organic chem, and nearly failing my classes. but those tatoos are the skeletal formula. each corner represents a carbon atom. the secondary lines represent double bonds. the other letters are other atoms in the molecule
for example. bottom left carbon is double bonded to oxygen, and has one bond to the adjacent nitrogens
i think this is some form of cycloalkene but i have no clue how to name it
princess pancake 

natty@astolfo.social
@sleepybisexual @iro_miya it’s caffeine
There’s also this nice tool where you can draw molecules and search by structure https://www.chemspider.com/structuresearch
sodiboo 
sodiboo@gaysex.cloud
@iro_miya it’s a skeletal formula where each atom has (roughly) up to 4 “bonds” with other atoms. some atoms have fewer. each vertex represents an atom. for what the letters mean, refer to the periodic table. each line represents a bond, and if there are two or more lines between vertices, it means there are two or more bonds between those two atoms.
C is carbon, and it binds 4 other atoms and is very common in many molecules because of this property.
N is nitrogen, and it binds 3 other atoms.
O is oxygen, and it binds 2 other atoms.
H is hydrogen, and it only binds 1 other atom, and is very common in many molecules because of this property.
to explain the precise rules of how each atom interacts and to make this all intuitive would need a chemistry course. also it’s not what you asked about. also I’m not super knowledgeable. but the above is the basics that matter most.
because C and H are so common, their labels are optional. if there is no letter on a vertex, it is either C (carbon), or H (hydrogen). count the number of lines from that vertex to know which.
also, these are 3D structures. each “4 bonds” will spread out in this shape. sometimes a molecule is symmetric so you’ll see just 4 equal lines eliminating from an unlabeled (C) vertex, but if the direction matters, then there will be a vertex that has one solid bold triangular line going “away” from you, and one lighter partial triangular line going “towards” you. here’s an example. each “3 bonds” will spread out into the same shape but missing one limb. in that case, when it matters, there will just be one of those triangular lines (or maybe both with just one normal line?). each “2 bonds” will again spread into a similar shape; this is why every vertex with two lines always has them 120°(-ish) apart.
also, it’s okay to put entire groups of atoms onto a vertex. like “OH” is a very common one; this is shorthand for “a vertex with an O, and another line connecting to an H”. chemists are very lazy and will not want to draw the entire structure in this 3D; and especially if it has lots of these unambiguous leaf groups, they are usually uninteresting. so they’re collapsed like this.
sodiboo 
sodiboo@gaysex.cloud
@iro_miya how do you read them into like. a name. that you can easily search for and/or pronounce? idk take a chemistry class. the naming of molecules is an entire lecture or two with new conventions as you learn fancy new types of molecules. for a lot of the “linear” ones (with a chain of carbons and some stuff hanging off the side of each), you start in one end and just index each non-hydrogen attachment, ending with the name of the hydrocarbon that has that many carbon atoms (methane, ethane, butane, propane, pentane, hexane; first four are quirky). start in whichever end results in smaller indices I guess. how do you name each attachment? fuck if I know. I studied this shit and got an A but it’s so painful to learn. “OH” is just the suffix “-nol”. if the carbons make a ring you add the prefix “cyclo-“ I think. if they make two or more rings you pray. if there are two carbon chains I think that’s an ester so you concatenate them in lexical sorting order? if there are more chains then they didn’t teach me this in school. if it’s ever unambiguous to omit information, you always omit it. chemists are very ffic. never repeat yourself if the thing you’re about to say can be deduced from prior information. this keeps names short and sudoku-like.
⏣‑5556667 [Nivia] 
Override@mk.absturztau.be
@iro_miya “hmm, I wonder which molecule this is”
-> perhaps from the IUPAC name I would be able to tell
-> lets build the molecule
-> “build molecule online”
-> first link is (attention this is a spoiler) https://app.molview.com/
-> its the exact molecule in question and the title tells which molecule it is
Charlotte  /Cinny
/Cinny 



charlotte
@sodiboo @iro_miya this base structure is a purine which is raccounted raccounter-racclockwise from the leftmost nitrogen, then racclockwise from the northeast most nitrogen
it has a methyl group at 1, 3, 7
it has a keto groups at 2 and 6
1,3,7-trimethylpurine-2,6dione
(working backwards here mostly i am not an organic racchemist but this is definitely raccaffeine)
sodiboo 
sodiboo@gaysex.cloud
@charlotte @iro_miya yeah see. purine. ive never heard of this before. there’s so much shit you gotta memorize and the worst part is it doesn’t even feel unnecessary. molecules genuinely just come in a lot of shapes and it’s difficult to name them regularly and unambiguously. if you’ve never encountered a certain base structure before you just don’t know. you have to just know. god i hate studying chemistry not because it’s a bullshit subject; all of this feels relatively logical but there’s just So Much Of It 
sincerely,
~ a burnt out chemistry student.
fluffboy 

goes2hard@labyrinth.zone
https://pubchem.ncbi.nlm.nih.gov/compound/2519
julia
juliaaa@chaos.socialAntifa Ost Sparkassenedition
transcaffeine@mond-basis.eu
 Anya
Anya 
anya@is.acute.cat
@iro_miya Unhelpful answer, but for me this is just a memory item. Oh yeah, that’s probably caffeine and if not definitely some sort of xanthine alkaloid with stimulant properties. Or maybe it kills you. Or both. :3
alina🐾💖✨🏳️⚧️
alina@girldick.gay@iro_miya IUPAC nomenclature
https://en.wikipedia.org/wiki/IUPAC_nomenclature_of_organic_chemistry